A Carbohydrate That Reaches the Colon Intact
Galactooligosaccharides (GOS) are non-digestible short-chain carbohydrates made of galactose units linked to a terminal glucose via β(1–3)-, β(1–4)-, or β(1–6)-glycosidic bonds. They are typically enzymatically synthesized from lactose using β-galactosidase, yielding chains of 2 to 8 sugar units.
Although technically susceptible to hydrolysis by human intestinal β-galactosidase, this activity is minimal. As a result, GOS travel intact through the small intestine to the colon, where their biological significance begins.
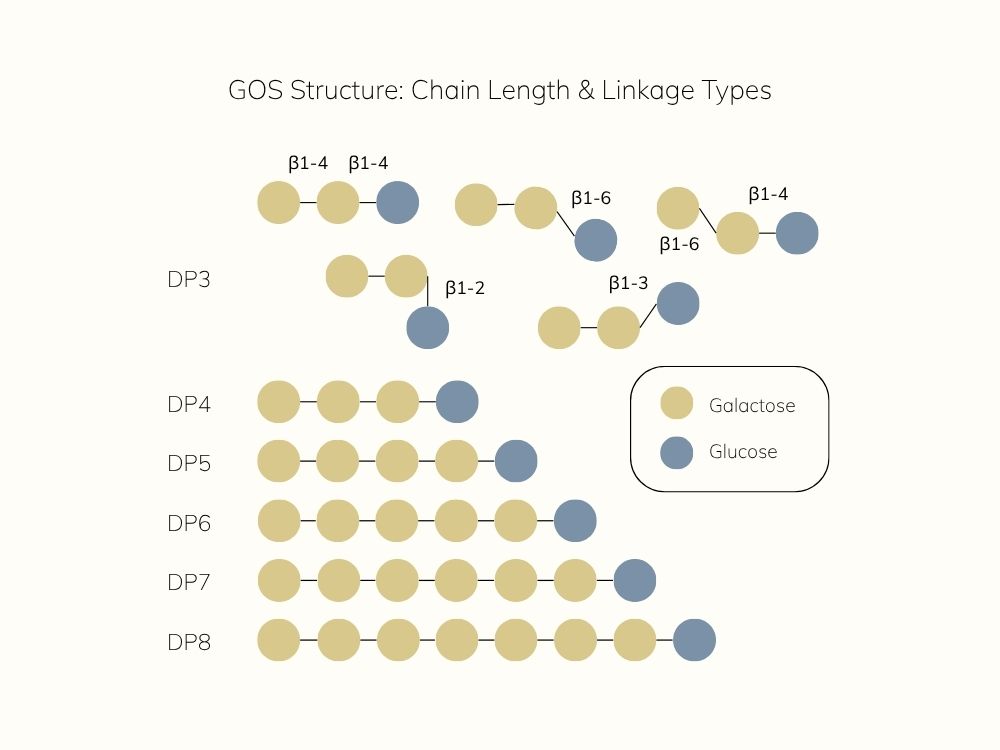
β-GOS can vary in both chain length (degree of polymerization, DP) and glycosidic linkage type.
Shorter chains like DP3 often feature mixed linkages, e.g. β(1→3), β(1→4), or β(1→6), while longer chains (DP4–DP8) are typically linear β(1→4).
These structural nuances influence fermentability, microbial selectivity, and site of action in the gut.
A Selective Feed for Key Microbial Players
Once in the colon, GOS serve as fermentable substrates for specific gut microbes. The main ones are Bifidobacterium species, which possess the enzymatic toolkit to utilize β-linked galactose structures. This selective stimulation is known as the bifidogenic effect [1].
Supplementation with GOS not only increases Bifidobacterium abundance [1,2], but it has also been shown to boost butyrate-producing species like Faecalibacterium prausnitzii, Coprococcus comes [1], and Eubacterium rectale [2], all of which contribute to colonic and systemic health. A concurrent reduction in Bacteroides species, often associated with Western dietary patterns, has also been observed [1,2].
Additionally, GOS supports the growth of Lactobacillus species, particularly L. gasseri and L. salivarius [2,3], and has been shown to inhibit the adhesion of toxins and pathogenic E. coli and Salmonella strains to epithelial cells [4,5].
Structure Matters: Why GOS Works the Way It Does
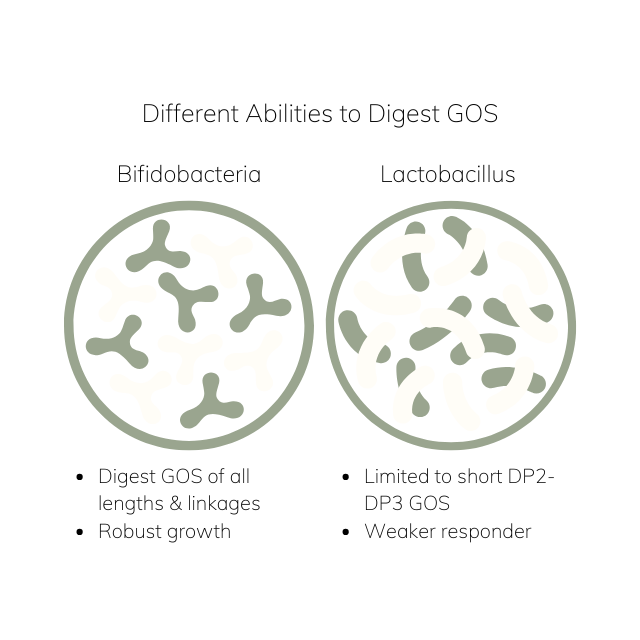
The prebiotic effect of GOS depends heavily on its structural diversity. In one study, the ability of 13 commercial probiotic lactic acid bacteria (LAB) and bifidobacteria to digest 40 different GOS compounds. Lactobacillus acidophilus and Bifidobacterium infantis demonstrated the broadest utilization range, fermenting 38 and 36 compounds, respectively [6].
In general, they found that Bifidobacterium can metabolize even branched GOS with higher degrees of polymerization, while most LAB strains prefer shorter-chain GOS, especially disaccharides [6]. This versatility in utilization helps explain the wide-reaching yet selective modulation of the microbiota seen with GOS supplementation.

Interlude: GOS Has a Sibling, Meet α-GOS
Not all galactooligosaccharides are created equal. While the GOS in AB INTRA formulations are structurally similar to the oligosaccharides found in mammalian breast milk and are produced synthetically from lactose using β-galactosidases, their “sibling” α-GOS comes from a different source and with different traits.
α-GOS occur naturally in foods like soybeans and legumes. They include raffinose, stachyose, and verbascose, galactose units linked α(1–6) to the glucose portion of sucrose. Because most mammals, including humans, lack pancreatic α-galactosidase, these fibers pass undigested into the colon, where colonic bacteria take over.
The difference? Fermentation of α(1–6) GOS often produces more fermentative gases, which in some people can cause abdominal discomfort, bloating, or flatulence. In contrast, GOS are generally well-tolerated, making them a more targeted choice for those seeking prebiotic benefits without excessive GI distress.
GOS in the Colon: Site-Specific Fermentation
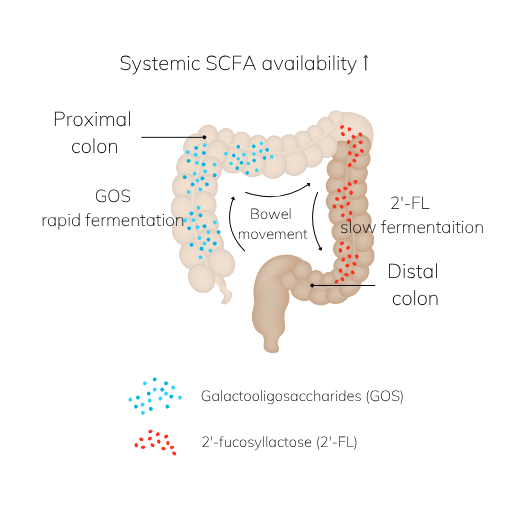
Unlike broad-spectrum fibers, GOS exert precise effects due to their fermentability and molecular structure. In the proximal colon, GOS are rapidly fermented, leading to the production of short-chain fatty acids (SCFAs), primarily acetate [3].
When paired with 2’-fucosyllactose (2’-FL), a human milk oligosaccharide analogue, this combination can extend their reach. GOS acts in the proximal colon; 2’-FL, in the distal colon [7,8]. This pairing enables a broader coverage of the gut, which may enhance systemic SCFA availability.
SCFAs produced in the distal colon are more likely to bypass first-pass liver metabolism via rectal veins, entering systemic circulation through the vena cava [9], which provides beneficial physiological effects [10,11]. This distinction makes spatially targeted SCFA production an important strategy in optimizing metabolic and immunological outcomes.
Beyond Bifidogenic
Although Bifidobacteria are primary responders to GOS, these oligosaccharides support wider microbial modulation. Certain Bifidobacterium strains that struggle to utilize 2’-FL alone benefit from the presence of GOS, making the combination especially effective in multi-substrate formulations.
In Gutbiotica CORE, GOS is paired with 2’-FL and human-residential Bifidobacterium strains to support both microbial diversity and functional metabolite output, especially short-chain fatty acids that are pivotal to gut, systemic and skin health.
Gut Comfort, Backed by Research
Beyond microbiome modulation, GOS also offer tangible digestive benefits, particularly for individuals with sensitive guts.
Several studies have shown that galactooligosaccharides help reduce common gastrointestinal symptoms, such as bloating, abdominal discomfort, and urgency:
- In healthy individuals, GOS intake significantly reduced flatulence, bloating, and abdominal pain, supporting overall digestive ease [12].
- In individuals with Irritable Bowel Syndrome (IBS), GOS supplementation improved GI symptoms, including reduced bloating and pain, without triggering discomfort [13,14].
- Notably, GOS were better tolerated than inulin-type fructans, which often worsen symptoms in IBS patients [15].
These findings suggest that GOS are a gut-friendly fiber option, even for those with digestive sensitivities, offering prebiotic benefits without the bloating baggage.
GOS and the Phenol Problem
Not all microbial metabolites benefit the host. Phenols, including phenol and p-cresol, are produced from aromatic amino acids by certain gut bacteria under suboptimal conditions (e.g. higher gut pH) [16-18]. These metabolites are often considered biomarkers of dysbiosis.
Phenols can enter systemic circulation and accumulate in the skin, where they’ve been shown to impair keratinocyte differentiation in murine models [19]. Their presence is associated with skin dryness and barrier disruption.
GOS offers a microbiome-mediated solution. In clinical studies, GOS intake reduced phenol production by fostering beneficial microbial activity. In women, it prevented transepidermal water and keratin loss associated with phenol exposure [20]. In animal studies, GOS prevented the development of atopic dermatitis by promoting IL-10 and inhibiting IL-17, two cytokines involved in inflammation [21].
Notably, the combination of GOS and Bifidobacteria, such as those in Skinbiotica COLLAGEN+, has been shown to enhance these skin benefits by reducing phenol load and modulating inflammatory responses [19,20,22].
References
- L. M. G. Davis, I. Martínez, J. Walter, C. Goin, and R. W. Hutkins, “Barcoded Pyrosequencing Reveals That Consumption of Galactooligosaccharides Results in a Highly Specific Bifidogenic Response in Humans,” PLoS One, vol. 6, no. 9, p. e25200, Sep. 2011. https://doi.org/10.1371/journal.pone.0025200
- J. Vulevic, A. Drakoularakou, P. Yaqoob, G. Tzortzis, and G. R. Gibson, “Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers,” Am. J. Clin. Nutr., vol. 88, no. 5, pp. 1438–1446, 2008, doi: https://doi.org/10.3945/ajcn.2008.26242.
- A. J. H. Maathuis, E. G. van den Heuvel, M. H. C. Schoterman, and K. Venema, “Galacto-Oligosaccharides Have Prebiotic Activity in a Dynamic In Vitro Colon Model Using a 13C-Labeling Technique,” J. Nutr., vol. 142, no. 7, pp. 1205–1212, Jul. 2012, doi: 10.3945/jn.111.157420.
- A. Monteagudo-Mera, R. A. Rastall, G. R. Gibson, D. Charalampopoulos, and A. Chatzifragkou, “Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health,” Appl. Microbiol. Biotechnol., vol. 103, no. 16, pp. 6463–6472, 2019, doi: 10.1007/s00253-019-09978-7.
- L. E. J. Searle et al., “A mixture containing galactooligosaccharide, produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium infection in mice,” J. Med. Microbiol., vol. 58, no. 1, 2009, doi: https://doi.org/10.1099/jmm.0.004390-0.
- Böger, S. S. van Leeuwen, A. Lammerts van Bueren, and L. Dijkhuizen, “Structural Identity of Galactooligosaccharide Molecules Selectively Utilized by Single Cultures of Probiotic Bacterial Strains,” J. Agric. Food Chem., vol. 67, no. 50, pp. 13969–13977, Dec. 2019, doi: 10.1021/acs.jafc.9b05968.
- P. Van den Abbeele, C. Duysburgh, E. Vazquez, J. Chow, R. Buck, and M. Marzorati, “2′-Fucosyllactose alters the composition and activity of gut microbiota from formula-fed infants receiving complementary feeding in a validated intestinal model,” J. Funct. Foods, vol. 61, p. 103484, 2019, doi: https://doi.org/10.1016/j.jff.2019.103484.
- R. Akkerman et al., “Combining galacto-oligosaccharides and 2′-fucosyllactose alters their fermentation kinetics by infant fecal microbiota and influences AhR-receptor dependent cytokine responses in immature dendritic cells,” Food Funct., vol. 13, no. 12, pp. 6510–6521, 2022, doi: 10.1039/D2FO00550F.
- E. P. J. G. Neis et al., “Distal versus proximal intestinal short-chain fatty acid release in man,” Gut, vol. 68, no. 4, pp. 764 LP – 765, Apr. 2019, doi: 10.1136/gutjnl-2018-316161.
- C. M. van der Beek et al., “Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men,” Clin. Sci., vol. 130, no. 22, pp. 2073–2082, Oct. 2016, doi: 10.1042/CS20160263.
- E. E. Canfora et al., “Supplementation of Diet With Galacto-oligosaccharides Increases Bifidobacteria, but Not Insulin Sensitivity, in Obese Prediabetic Individuals,” Gastroenterology, vol. 153, no. 1, pp. 87-97.e3, 2017, doi: https://doi.org/10.1053/j.gastro.2017.03.051.
- J. Vulevic, G. Tzortzis, A. Juric, and G. R. Gibson, “Effect of a prebiotic galactooligosaccharide mixture (B-GOS®) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence,” Neurogastroenterol. Motil., vol. 30, no. 11, p. e13440, Nov. 2018, doi: https://doi.org/10.1111/nmo.13440.
- J.-W. Huaman et al., “Effects of Prebiotics vs a Diet Low in FODMAPs in Patients With Functional Gut Disorders,” Gastroenterology, vol. 155, no. 4, pp. 1004–1007, 2018, doi: https://doi.org/10.1053/j.gastro.2018.06.045.
- D. B. A. SILK, A. DAVIS, J. VULEVIC, G. TZORTZIS, and G. R. GIBSON, “Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome,” Aliment. Pharmacol. Ther., vol. 29, no. 5, pp. 508–518, Mar. 2009, doi: https://doi.org/10.1111/j.1365-2036.2008.03911.x.
- B. Wilson, M. Rossi, E. Dimidi, and K. Whelan, “Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials,” Am. J. Clin. Nutr., vol. 109, no. 4, pp. 1098–1111, 2019, doi: https://doi.org/10.1093/ajcn/nqy376.
- R. Iizuka, K. Koji, I. Naoki, and K. and Chiba, “Phenols produced by gut bacteria affect the skin in hairless mice,” Microb. Ecol. Health Dis., vol. 21, no. 1, pp. 50–56, Jan. 2009, doi: 10.1080/08910600802688910.
- E. A. Smith and G. T. Macfarlane, “Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism,” J. Appl. Bacteriol., vol. 81, no. 3, pp. 288–302, Sep. 1996, doi: https://doi.org/10.1111/j.1365-2672.1996.tb04331.x.
- V. De Preter et al., “The in vivo use of the stable isotope-labelled biomarkers lactose-[15N]ureide and [2H4]tyrosine to assess the effects of pro- and prebiotics on the intestinal flora of healthy human volunteers,” Br. J. Nutr., vol. 92, no. 3, pp. 439–446, 2004, doi: DOI: 10.1079/BJN20041228.
- K. Miyazaki, N. Masuoka, M. Kano, and R. Iizuka, “Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota,” Benef. Microbes, vol. 5, no. 2, pp. 121–128, 2014, doi: https://doi.org/10.3920/BM2012.0066.
- M. KANO et al., “Consecutive Intake of Fermented Milk Containing Bifidobacterium breve Strain Yakult and Galacto-oligosaccharides Benefits Skin Condition in Healthy Adult Women,” Biosci. Microbiota, Food Heal., vol. 32, no. 1, pp. 33–39, 2013, doi: 10.12938/bmfh.32.33.
- S. Tanabe and S. Hochi, “Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice,” Int J Mol Med, vol. 25, no. 3, pp. 331–336, 2010, doi: 10.3892/ijmm_00000349.
- K.-B. Hong, J. Mingeum, H. Ki Soo, H. K. Jae, P. Yooheon, and H. J. and Suh, “Photoprotective effects of galacto-oligosaccharide and/or Bifidobacterium longum supplementation against skin damage induced by ultraviolet irradiation in hairless mice,” Int. J. Food Sci. Nutr., vol. 66, no. 8, pp. 923–930, Nov. 2015, doi: 10.3109/09637486.2015.1088823.
Next in Your Gut Health Journey
Explore further the fascinating world of gut health. Because better health is built one insight at a time.
Where Your Gut Meets Science
€ 269,85 Original price was: € 269,85.€ 214,95Current price is: € 214,95. incl. VAT
Consistency meets radiance. A 3-month supply of Skinbiotica COLLAGEN+—science-backed skin nutrition to support skin collagen, elasticity, and glow from within.
€ 209,90 Original price was: € 209,90.€ 179,95Current price is: € 179,95. incl. VAT
Complete beauty from within—Gutbiotica PRIME provides comprehensive microbiome support while Skinbiotica COLLAGEN+ promotes radiant and elastic skin.
€ 164,90 Original price was: € 164,90.€ 139,95Current price is: € 139,95. incl. VAT
A smart pairing for gut microbiome and weight support with Gutbiotica CORE and Fibersentials SHAPE to maintain a happy gut and support satiety naturally.