Small but Mighty: What Are Short-Chain Fatty Acids?
Short-chain fatty acids (SCFAs) are small but mighty compounds your gut bacteria produce when they ferment dietary fiber. They may be microscopic in size, but their effects are anything but small.
Of all SCFAs, three dominate:
- Acetate (~60%)
- Propionate (15–25%)
- Butyrate (15–25%) [1].
These molecules are mostly created in your proximal colon and absorbed rapidly by colonocytes, fueling gut cells and contributing up to 10% of your daily energy needs [2].
They are messengers, shields, and energy sources, helping regulate everything from digestion to immunity to mood.
Where Do Short-Chain Fatty Acids Come From?
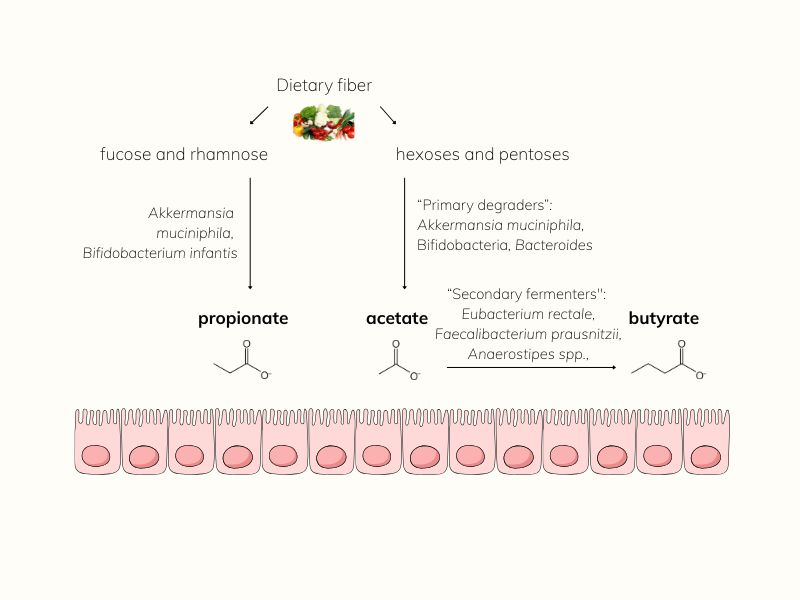
It all begins with fiber, the kind your body can’t digest, but your gut microbes can.
When you eat fermentable fibers like resistant starches, inulin, galacto-oligosaccharides (GOS), and 2’-fucosyllactose (2’-FL), your microbiome gets to work. But this process isn’t simple. Fiber fermentation is a multi-step collaboration involving different microbial players, each with a distinct role.
Microbial Harmony: Primary Degraders and Cross-Feeders
Primary degraders or “keystone species” break down complex fibers using specialized enzymes [3].
Cross feeders or “secondary fermenters” thrive on their by-products, transforming them into SCFAs.
Together, they create the rich pool of short-chain fatty acids (SCFAs) that nourish your gut and beyond.
From fiber to fuel: The microbial pathway to SCFAs
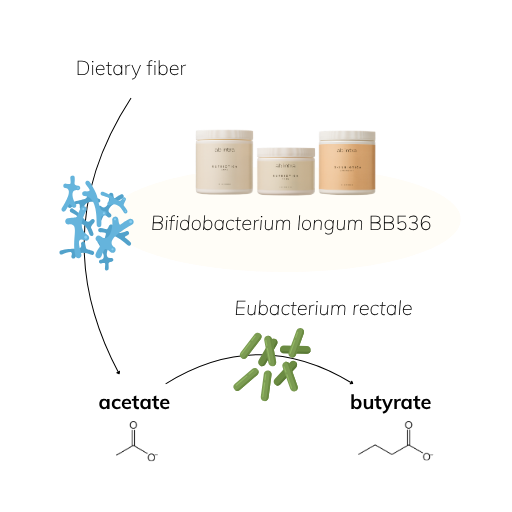
A Closer Look: BB536 & the Power of Microbial Teamwork
Take Bifidobacterium longum BB536, one of the clinically studied strains we use in Gutbiotica CORE, Gutbiotica PRIME, and Skinbiotica COLLAGEN+. In fermentation processes, BB536 produces acetate, which in turn fuels the growth of other beneficial microbes, such as Eubacterium rectale, that convert acetate and lactate into butyrate, a key SCFA for gut lining support [4,5]. This is microbial teamwork in action: your beneficial bacteria don’t just survive—they collaborate, cross-feed, and amplify one another’s effects.
Mapping the Microbial Cast
Each fiber you eat sparks a different microbial response. Here’s a glimpse at who’s working behind the scenes:
- Bifidobacteria, Bacteroides, and Prevotella produce acetate and lactate.
- Firmicutes, such as Faecalibacterium, Roseburia, Eubacterium, and Anaerostipes, transform these into butyrate.
- Akkermansia muciniphila also produces acetate, but it stands out as the major propionate producer [6].
The type of fiber you consume and the composition of your gut microbiota determine not just how much SCFA is produced, but which types and in what proportions [7,8]. That’s why our metabolic response to fiber is so personal, uniquely shaped by your microbial fingerprint.
In this way, your microbiome turns what your body can’t digest into something profoundly nourishing. A molecular gift, created from synergy, science, and the simple act of eating well.
5 Powerful Benefits of SCFAs
1. They Fortify Your Gut Lining
Butyrate is the preferred fuel of your colon cells, providing up to 70% of their energy [2]. It helps strengthen your gut barrier by:
- Increasing tight junction proteins (claudin-1, ZO-1, occludin) [9,10]
- Activating HIF-1α, which is protective to gut barrier integrity [11,12]
- Stimulating the production of mucin (MUC2) by Goblet cells, thickening the gut’s protective mucus layer [13]
In short: SCFAs keeps your barrier strong, reducing permeability (aka “leaky gut”) and resilient.
2. They Soothe Inflammation
Butyrate and propionate have powerful anti-inflammatory properties. Butyrate:
- Inhibits histone deacetylases (HDACs) in macrophages, one of immune cells macrophages [14,15].
- Regulates inflammatory signaling pathways like NF-κB [14,15].
- Promotes regulatory T cells that prevent immune overreaction [16].
This immune-calming effect has broad relevance, from autoimmunity to skin conditions to brain health.
3. They Support Metabolic Balance
SCFAs play a direct role in appetite, energy regulation, and blood sugar control:
- Activate FFAR2/3 receptors in gut cells to release PYY and GLP-1, key satiety hormones [17]
- Delay gastric emptying and reduce hunger signals via hypothalamic circuits [18,19]
- Influence fat storage and insulin sensitivity through leptin modulation [20]
Your body has evolved to produce these signals naturally from fiber.

Interlude: GLP-1, Nature’s Satiety Signal
Heard of GLP-1? It’s the same hormone weight-loss drugs like Ozempic are built to mimic.
When released naturally, GLP-1 signals your body to slow down eating, feel full, delay gastric emptying and begin nutrient absorption. The effect is quick, but short-lived. That’s where GLP-1 agonist drugs differ: they extend this signal for days, often eliminating hunger altogether.
But here’s the good news: you don’t need a prescription to benefit from GLP-1.
Fermentable fiber is nature’s way of supporting GLP-1 production—gradually, gently, and in sync with your body. By feeding your gut microbes, you help your intestine keep GLP-1 flowing, supporting satiety and metabolic balance.
More fiber. More fullness. From Within.
4. They Shape Your Mood and Brain Health
SCFAs are key players in the gut-brain axis. They help:
- Modulate serotonin, GABA, and dopamine levels [21]
- Protect against neuroinflammation by regulating immune signaling [22]
- Influence memory and learning via pathways like NMDAR activation [23,24]
In human studies, SCFAs have even been shown to reduce cortisol response to stress [25].
5. They Maintain a Balanced Microbial Environment
Butyrate not only feeds your gut cells, it also shapes the microbial landscape:
- Promotes anaerobic conditions that favor beneficial species [26]
- Activates PPARγ in colonocytes, reducing oxygen and nitrate levels, which are pathogenic bacteria exploit [26]
By supporting a low-oxygen, fiber-fueled environment, SCFAs help keep your microbiome stable and self-regulating.
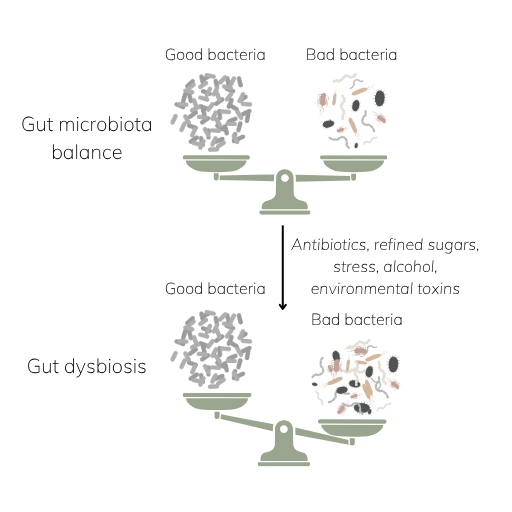
What Can Disrupt SCFA Production?
Short-chain fatty acids don’t just appear. They’re the product of microbial teamwork. To make them, your gut needs the right inputs (dietary fiber) and the right microbes to ferment it.
When either is missing, whether it’s from a low-fiber diet or an imbalanced microbiome, SCFA production drops. In fact, the same factors that drive gut dysbiosis often impair your microbiome’s ability to generate these vital compounds.
Some of the most common disruptors include:
- Low-fiber, highly processed diets [27]
- Frequent antibiotic use [28]
- Chronic stress [29]
- Reduced diversity (common with age) [30]
- Inflammation and gut dysbiosis [31]
When this microbial balance breaks, SCFA output drops and so does the protective support your gut (and body) depends on.
How to Support SCFA Production
Diet is one of the most powerful drivers of your microbiome’s composition and what it produces [32,33]. Here’s how to help your microbes do what they do best:
- Feed Them Fiber
Include fermentable fibers like GOS, inulin, pectin, β-glucan, resistant starch, and 2’-FL. Found in whole-grain foods, legumes, fruits and vegetables, and AB INTRA formulations. Evidence shows that going even higher, toward ~45 g/day, can increase SCFA production and promotes the activity of fiber-degrading enzymes in the gut microbiome [34,35]. - Add Prebiotics + Probiotics
Pair the right fuel with the right strains.
Gutbiotica CORE combines GOS + 2’-FL to support a range of Bifidobacteria and SCFA-producing pathways.
PRIME builds on this with age-conscious probiotic strains, while Fibersentials FLOW and SHAPE provide extra fermentable fibers to amplify the effect. - Eat a Diverse, Microbe-Friendly Diet
Fiber variety fuels species variety. The more diverse your plant intake, the more robust your microbial community. Add fermented foods too, your gut loves them.
And don’t forget: physical activity, circadian rhythm alignment, and stress management all influence microbial stability—and the production of short-chain fatty acids.
A Final Word: You Feed Them, They Feed You
SCFAs are not just leftovers from fiber fermentation. They’re gifts from your microbes, small molecules with far-reaching benefits.
At AB INTRA, we formulate with SCFAs support in mind. From Gutbiotica’s daily foundation to Fibersentials’ dietary fiber blend, our gut health supplements are designed to nourish the inner ecosystem that nourishes you.
Because when your gut thrives, so do you.
References
- L. Topping and P. M. Clifton, “Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides,” Physiol. Rev., vol. 81, no. 3, pp. 1031–1064, Jul. 2001, doi: 10.1152/physrev.2001.81.3.1031.
- G. den Besten, K. van Eunen, A. K. Groen, K. Venema, D.-J. Reijngoud, and B. M. Bakker, “The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism,” J. Lipid Res., vol. 54, no. 9, pp. 2325–2340, Sep. 2013, doi: 10.1194/jlr.R036012.
- H. J. Flint, K. P. Scott, P. Louis, and S. H. Duncan, “The role of the gut microbiota in nutrition and health,” Nat. Rev. Gastroenterol. Hepatol., vol. 9, no. 10, pp. 577–589, 2012, doi: 10.1038/nrgastro.2012.156.
- F. Gwen, V. Angeliki, V. Kristof, and V. L. De, “Cross-Feeding between Bifidobacterium longum BB536 and Acetate-Converting, Butyrate-Producing Colon Bacteria during Growth on Oligofructose,” Appl. Environ. Microbiol., vol. 72, no. 12, pp. 7835–7841, Dec. 2006, doi: 10.1128/AEM.01296-06.
- H. Sugahara et al., “Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community,” Sci. Rep., vol. 5, no. 1, p. 13548, 2015, doi: 10.1038/srep13548.
- B. Clara et al., “Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B12 Production by Intestinal Symbionts,” MBio, vol. 8, no. 5, pp. 10.1128/mbio.00770-17, Sep. 2017, doi: 10.1128/mbio.00770-17.
- J. Yang, I. Martínez, J. Walter, A. Keshavarzian, and D. J. Rose, “In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production,” Anaerobe, vol. 23, pp. 74–81, 2013, doi: https://doi.org/10.1016/j.anaerobe.2013.06.012.
- J. Yang and D. J. Rose, “Long-term dietary pattern of fecal donor correlates with butyrate production and markers of protein fermentation during in vitro fecal fermentation,” Nutr. Res., vol. 34, no. 9, pp. 749–759, 2014, doi: https://doi.org/10.1016/j.nutres.2014.08.006.
- T. Van Hung and T. Suzuki, “Dietary Fermentable Fibers Attenuate Chronic Kidney Disease in Mice by Protecting the Intestinal Barrier,” J. Nutr., vol. 148, no. 4, pp. 552–561, 2018, doi: https://doi.org/10.1093/jn/nxy008.
- D. Parada Venegas et al., “Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases,” Front. Immunol., vol. Volume 10, 2019.
- C. J. Kelly et al., “Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function,” Cell Host Microbe, vol. 17, no. 5, pp. 662–671, 2015, doi: https://doi.org/10.1016/j.chom.2015.03.005.
- L. P. Pral, J. L. Fachi, R. O. Corrêa, M. Colonna, and M. A. R. Vinolo, “Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions,” Trends Immunol., vol. 42, no. 7, pp. 604–621, Jul. 2021, doi: 10.1016/j.it.2021.05.004.
- E. Gaudier et al., “Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose,” Am. J. Physiol. Liver Physiol., vol. 287, no. 6, pp. G1168–G1174, Dec. 2004, doi: 10.1152/ajpgi.00219.2004.
- W. Ratajczak, A. Rył, A. Mizerski, K. Walczakiewicz, O. Sipak, and M. Laszczyńska, “Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs),” Acta Biochim. Pol., vol. 66, no. 1, pp. 1–12, 2019, doi: 10.18388/abp.2018_2648.
- M. Kasubuchi, S. Hasegawa, T. Hiramatsu, A. Ichimura, and I. Kimura, “Dietary Gut Microbial Metabolites, Short-chain Fatty Acids, and Host Metabolic Regulation,” Nutrients, vol. 7, no. 4. pp. 2839–2849, 2015, doi: 10.3390/nu7042839.
- Y. Furusawa et al., “Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells,” Nature, vol. 504, no. 7480, pp. 446–450, 2013, doi: 10.1038/nature12721.
- A. Puddu, R. Sanguineti, F. Montecucco, and G. L. Viviani, “Evidence for the Gut Microbiota Short-Chain Fatty Acids as Key Pathophysiological Molecules Improving Diabetes,” Mediators Inflamm., vol. 2014, no. 1, p. 162021, Jan. 2014, doi: https://doi.org/10.1155/2014/162021.
- A. De Silva and and S. R. Bloom, “Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity,” Gut Liver, vol. 6, no. 1, pp. 10–20, Jan. 2012, doi: 10.5009/gnl.2012.6.1.10.
- E. Näslund et al., “GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans,” Am. J. Physiol. Integr. Comp. Physiol., vol. 277, no. 3, pp. R910–R916, Sep. 1999, doi: 10.1152/ajpregu.1999.277.3.R910.
- S. H. Al-Lahham et al., “Regulation of adipokine production in human adipose tissue by propionic acid,” Eur. J. Clin. Invest., vol. 40, no. 5, pp. 401–407, May 2010, doi: https://doi.org/10.1111/j.1365-2362.2010.02278.x.
- G. Frost et al., “The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism,” Nat. Commun., vol. 5, no. 1, p. 3611, 2014, doi: 10.1038/ncomms4611.
- Kirsten Berding, Carina Carbia, and John F Cryan, “Going with the grain: Fiber, cognition, and the microbiota-gut-brain-axis,” Exp. Biol. Med., vol. 246, no. 7, pp. 796–811, Feb. 2021, doi: 10.1177/1535370221995785.
- B. Gronier et al., “Increased cortical neuronal responses to NMDA and improved attentional set-shifting performance in rats following prebiotic (B-GOS®) ingestion,” Eur. Neuropsychopharmacol., vol. 28, no. 1, pp. 211–224, 2018, doi: https://doi.org/10.1016/j.euroneuro.2017.11.001.
- A. C.-C. Kao, S. Spitzer, D. C. Anthony, B. Lennox, and P. W. J. Burnet, “Prebiotic attenuation of olanzapine-induced weight gain in rats: analysis of central and peripheral biomarkers and gut microbiota,” Transl. Psychiatry, vol. 8, no. 1, p. 66, 2018, doi: 10.1038/s41398-018-0116-8.
- B. Dalile, B. Vervliet, G. Bergonzelli, K. Verbeke, and L. Van Oudenhove, “Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: a randomized, placebo-controlled trial,” Neuropsychopharmacology, vol. 45, no. 13, pp. 2257–2266, 2020, doi: 10.1038/s41386-020-0732-x.
- W. M. de Vos, H. Tilg, M. Van Hul, and P. D. Cani, “Gut microbiome and health: mechanistic insights,” Gut, vol. 71, no. 5, pp. 1020 LP – 1032, May 2022, doi: 10.1136/gutjnl-2021-326789.
- E. D. Sonnenburg and J. L. Sonnenburg, “Starving our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates,” Cell Metab., vol. 20, no. 5, pp. 779–786, Nov. 2014, doi: 10.1016/j.cmet.2014.07.003.
- M. Blaser, “Stop the killing of beneficial bacteria,” Nature, vol. 476, no. 7361, pp. 393–394, 2011, doi: 10.1038/476393a.
- S.-J. Leigh et al., “The impact of acute and chronic stress on gastrointestinal physiology and function: a microbiota–gut–brain axis perspective,” J. Physiol., vol. 601, no. 20, pp. 4491–4538, Oct. 2023, doi: https://doi.org/10.1113/JP281951.
- T. S. Ghosh, F. Shanahan, and P. W. O’Toole, “The gut microbiome as a modulator of healthy ageing,” Nat. Rev. Gastroenterol. Hepatol., vol. 19, no. 9, pp. 565–584, 2022, doi: 10.1038/s41575-022-00605-x.
- M. Y. Zeng, N. Inohara, and G. Nuñez, “Mechanisms of inflammation-driven bacterial dysbiosis in the gut,” Mucosal Immunol., vol. 10, no. 1, pp. 18–26, 2017, doi: https://doi.org/10.1038/mi.2016.75.
- L. A. David et al., “Diet rapidly and reproducibly alters the human gut microbiome,” Nature, vol. 505, no. 7484, pp. 559–563, 2014, doi: 10.1038/nature12820.
- G. D. Wu et al., “Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes,” Science (80-. )., vol. 334, no. 6052, pp. 105–108, Oct. 2011, doi: 10.1126/science.1208344.
- H. C. Wastyk et al., “Gut-microbiota-targeted diets modulate human immune status,” Cell, vol. 184, no. 16, pp. 4137-4153.e14, Aug. 2021, doi: 10.1016/j.cell.2021.06.019.
- D. So et al., “Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis,” Am. J. Clin. Nutr., vol. 107, no. 6, pp. 965–983, 2018, doi: https://doi.org/10.1093/ajcn/nqy041.
Next in Your Gut Health Journey
Explore further the fascinating world of gut health. Because better health is built one insight at a time.
Where Your Gut Meets Science
€ 269,85 Original price was: € 269,85.€ 214,95Current price is: € 214,95. incl. VAT
Consistency meets radiance. A 3-month supply of Skinbiotica COLLAGEN+—science-backed skin nutrition to support skin collagen, elasticity, and glow from within.
€ 209,90 Original price was: € 209,90.€ 179,95Current price is: € 179,95. incl. VAT
Complete beauty from within—Gutbiotica PRIME provides comprehensive microbiome support while Skinbiotica COLLAGEN+ promotes radiant and elastic skin.
€ 164,90 Original price was: € 164,90.€ 139,95Current price is: € 139,95. incl. VAT
A smart pairing for gut microbiome and weight support with Gutbiotica CORE and Fibersentials SHAPE to maintain a happy gut and support satiety naturally.