- What Is Bifidobacterium longum BB536?
- Why BB536 Stands Out
- Proven Health Benefits of BB536
- BB536 Specialties: Microbial Conversation and Nutrient Synthesis
- Cross-Feeding and Strain Synergy: Why BB536 Doesn’t Work Alone
- BB536 in Gutbiotica PRIME: Supporting the Aging Microbiome
- In AB INTRA Formulations
- Conclusion: A Science-Backed Probiotic for Everyday Gut Health
Not all probiotics are created equal. While many offer general digestive support, only a select few are backed by robust scientific evidence. One such standout is Bifidobacterium longum BB536, a human-residential strain with decades of clinical research supporting its benefits for digestion, immunity, metabolism, and microbial balance.
Let’s explore what makes this strain exceptional, and why we’ve chosen it for key formulations at AB INTRA.
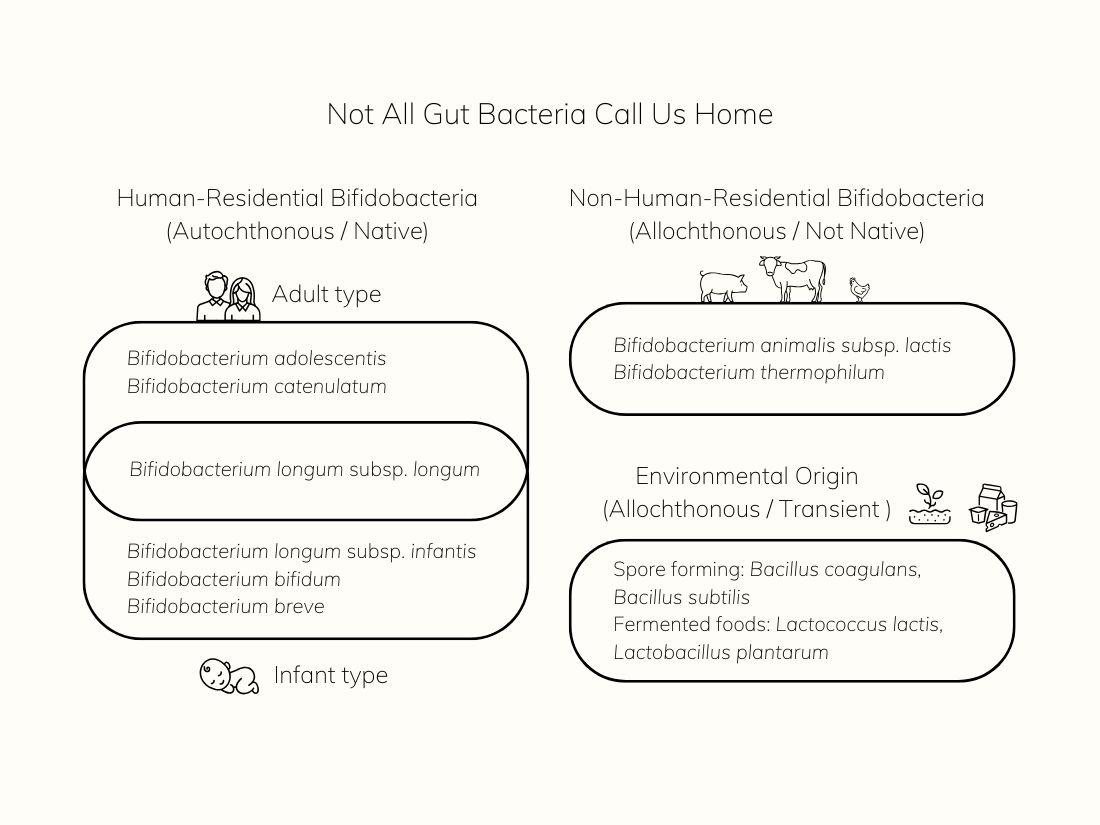
What Is Bifidobacterium longum BB536?
Bifidobacterium longum BB536 is a strain of Bifidobacterium longum subsp. longum, naturally found in the human gastrointestinal tract. It’s considered “autochthonous”, a native resident of the gut since the earliest stages of life. Unlike transient probiotic strains, BB536 is adapted to survive and interact within the human microbiome ecosystem.
Its resilience and compatibility stem from co-evolution with the host—your body—which means it’s more likely to establish, communicate with other beneficial microbes, and produce compounds your system understands.
The gut is a complex ecosystem. Some microbes have evolved with us, while others simply pass through. Understanding who stays and who visits helps us choose better support.
Native strains like human-residential Bifidobacteria are more likely to integrate with our microbiome, interact with our immune system, and contribute to long-term gut balance. Allochthonous strains may offer short-term support but often don’t stay.
Why BB536 Stands Out
1. Clinically Studied and Strain-Specific
Most probiotic benefits are strain-dependent, meaning not every strain of Bifidobacterium longum offers the same effects. BB536 has been tested thoroughly for its safety [1,2] and studied extensively for health benefits BB536 [3]:
- Gut health and regularity
- Immune system support
- Inflammatory response modulation
- Allergy relief
- Microbial balance under dietary stress
2. Human-Residential and Stable
Being autochthonous means Bifidobacterium longum is a bacterial species known to be a core member of the human gastrointestinal microbiota that forms stable and dominant populations in most individuals [4–6]. Studies confirm that native Bifidobacterium longum strains persist over time, offering enduring benefits [7,8].
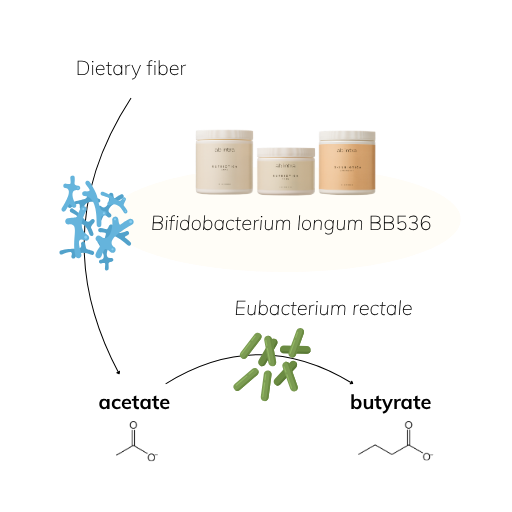
3. Specialist in Carbohydrate Fermentation
With a rich glycan-degrading gene pool, Bifidobacterium longum strains can digest a variety of oligosaccharides, including plant-based glycans and even human milk oligosaccharides (HMOs) [9]. Through fermentation, it produces key metabolites like acetate and lactate, fuel for other beneficial microbes like Eubacterium rectale, which in turn produce butyrate [10,11].
Proven Health Benefits of BB536
1. Digestive Comfort and Gut Regularity
BB536 helps promote regular bowel movements and eases gastrointestinal discomfort [12]. Research shows it can counteract disturbances from antibiotics and improve stool consistency [13], making your gut feel balanced and comfortable.
2. Microbial Balance Under Dietary Stress
Modern Western diets high in animal-based foods and low in fiber promote dysbiosis. BB536 helps restore balance by preventing the rise of inflammatory microbes such as Bilophila wadsworthia, a bacterium associated with animal-based diets and colorectal risk [14].
Interlude: The Rotten Egg Microbe
You may not know the name Bilophila wadsworthia, but you would recognise its calling card, the unmistakable rotten-egg smell of hydrogen sulfide. This gas, toxic in large amounts, is produced not just in drains and stagnant ponds, but also in small pockets of your gut.
Bilophila is a sulfate-reducing bacterium, yet unlike most of its kind, it can’t use sulfate. Instead, it feasts on taurine, which is abundant in meat and dairy, breaking it down into hydrogen sulfide. In small, controlled amounts, both the bacterium and the gas have their place, discouraging harmful microbes from settling in.
The trouble begins when Bilophila blooms. Too much hydrogen sulfide can irritate the gut lining, fuelling inflammation and increasing risks linked to inflammatory bowel disease, irritable bowel syndrome, and even colorectal cancer. Diets high in animal fat and low in fibre create the perfect environment for this overgrowth.
That’s where BB536 steps in, helping to stabilise the microbiome so that Bilophila remains a quiet background player, not a dominant force.
3. Immune Modulation and Inflammatory Control
Since up to 70% of your immune system lives in your gut, a balanced microbiome is essential. BB536 helps regulate cytokine production, reducing inflammation while boosting immune defenses. It’s been shown to improve mucosal barrier function in people with ulcerative colitis [15,16] and lower inflammatory markers post-colorectal surgery (e.g., IL-6 and procalcitonin) [17]. This makes it particularly beneficial for individuals with immune-related gut conditions or recovering from medical treatments.
4. Allergy Relief and Immune Balance
An imbalanced gut can trigger unwanted allergic reactions. BB536 helps restore the Th1/Th2 immune balance, a crucial factor in managing allergies [18]. In clinical studies, it lowered allergen-specific IgE and eosinophil levels, easing seasonal allergy symptoms and improving quality of life [19].

BB536 Specialties: Microbial Conversation and Nutrient Synthesis
This strain doesn’t work alone, it starts a conversation.
BB536 ferments complex carbohydrates into acetate and lactate [10], feeding other microbes like Eubacterium rectale, a key butyrate-producer [11]. This cross-feeding promotes a gut ecosystem that generates short-chain fatty acids (SCFAs), strengthens epithelial integrity, and nourishes colonocytes.
BB536 has also been linked to increased production of tryptophan metabolites such as indole-3-lactic acid (ILA), indole-3-acetic acid (IAA), and indole-3-aldehyde (IAld), all of which activate the aryl hydrocarbon receptor (AhR), modulating inflammation and enhancing gut-skin barrier function [20].
It also supports the growth of biotin-producing species like Bacteroides caccae, contributing to vitamin B7 production and metabolic resilience [11].
Cross-Feeding and Strain Synergy: Why BB536 Doesn’t Work Alone
In both Gutbiotica PRIME and Skinbiotica COLLAGEN+, BB536 is paired with one or more other human-residential Bifidobacterium strains. Why the blend?
Not all Bifidobacterium species are equal in their metabolic capabilities. While BB536 is a powerful strain in its own right, combining it with other complementary strains expands the scope of action. Metabolic specificity exists between bifidobacterial species, meaning different strains utilize different substrates and produce distinct metabolites. When combined, they create a richer environment for microbial interaction, including cross-feeding, a process where one strain’s metabolites become fuel for another [21] .
This trophic collaboration doesn’t just enhance microbial survival. It broadens the ecological niche of beneficial bacteria, making their presence more resilient and versatile under varying conditions [22].
BB536, for instance, plays a key role as a primary degrader of complex carbohydrates, including HMOs, but not all Bifidobacteria possess this upstream capacity [23]. Pairing BB536 with other strains that specialize in different substrates creates a cascading effect, supporting other beneficial microbes such as Eubacterium, which further metabolize BB536’s byproducts into butyrate and other short-chain fatty acids.
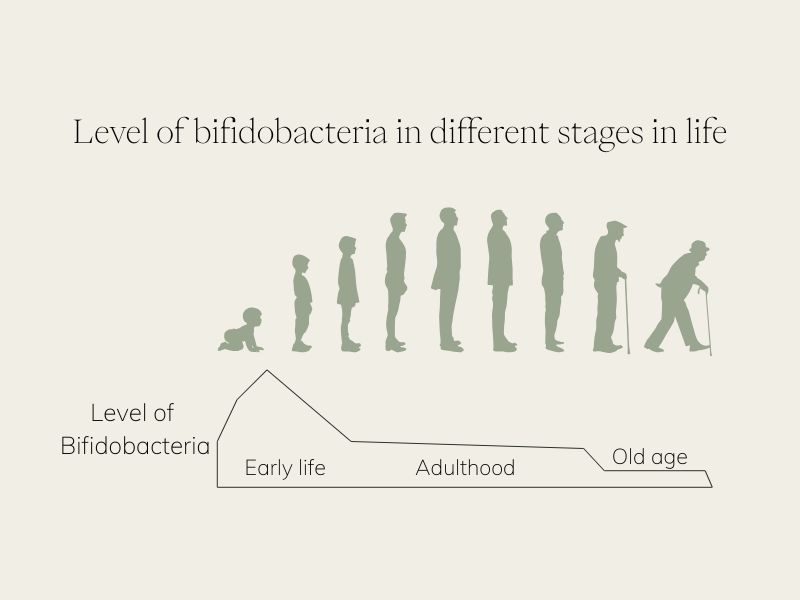
BB536 in Gutbiotica PRIME: Supporting the Aging Microbiome
Our reliable lifetime companion, Bifidobacteria, plays an essential role throughout the human lifespan, but their presence doesn’t remain constant. We begin life rich in Bifidobacteria, especially during infancy, but their abundance declines as we age [24], [25]. This microbial loss often coincides with a drop in SCFA-producing bacteria such as Faecalibacterium prausnitzii, Eubacterium, and Roseburia [26].
BB536 helps fill this gap. By producing lactate and acetate [21], it provides critical substrates that fuel downstream butyrate producers, reinforcing both gut integrity and systemic health.
In Gutbiotica PRIME, BB536 is combined with two other Bifidobacterium strains and 2’-FL, creating a targeted formulation for mature microbiomes. This trio of beneficial bacteria helps maintain microbial resilience, restore lost diversity, and sustain the SCFA production cascade that supports digestion, immune regulation, and metabolic balance in later life.
In AB INTRA Formulations
Bifidobacterium longum BB536 is a cornerstone of Gutbiotica CORE, Gutbiotica PRIME and Skinbiotica COLLAGEN+, paired with synergistic strains like B. breve M-16V and B. infantis M-63, and prebiotic substrates like galactooligosaccharides (GOS) and 2’-FL. Together they support:
- Gut barrier integrity
- Systemic immune balance
- Short-chain fatty acid (SCFA) production
- Gut-skin axis modulation
From internal calm to outer radiance, BB536 helps maintain balance where it matters most: from within.
Conclusion: A Science-Backed Probiotic for Everyday Gut Health
With its robust clinical foundation and deep evolutionary compatibility, Bifidobacterium longum BB536 is more than a probiotic. It’s a microbial ally shaped by co-existence with the human host. Whether you’re seeking digestive comfort, immune resilience, or whole-body support, BB536 offers a science-backed path to balance.
References
- Toscano, E. De Vecchi, A. Gabrieli, G. V. Zuccotti, and L. Drago, “Probiotic characteristics and in vitro compatibility of a combination of Bifidobacterium breve M-16 V, Bifidobacterium longum subsp. infantis M-63 and Bifidobacterium longum subsp. longum BB536,” Ann. Microbiol., vol. 65, no. 2, pp. 1079–1086, 2015, doi: 10.1007/s13213-014-0953-5.
- J. XIAO, S. TAKAHASHI, T. ODAMAKI, T. YAESHIMA, and K. IWATSUKI, “Antibiotic Susceptibility of Bifidobacterial Strains Distributed in the Japanese Market,” Biosci. Biotechnol. Biochem., vol. 74, no. 2, pp. 336–342, Feb. 2010, doi: 10.1271/bbb.90659.
- C. B. Wong, T. Odamaki, and J. Xiao, “Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action,” J. Funct. Foods, vol. 54, pp. 506–519, 2019, doi: https://doi.org/10.1016/j.jff.2019.02.002.
- I. Martínez, C. E. Muller, and J. Walter, “Long-Term Temporal Analysis of the Human Fecal Microbiota Revealed a Stable Core of Dominant Bacterial Species,” PLoS One, vol. 8, no. 7, p. e69621, Jul. 2013. https://doi.org/10.1371/journal.pone.0069621.
- S. Schloissnig et al., “Genomic variation landscape of the human gut microbiome,” Nature, vol. 493, no. 7430, pp. 45–50, 2013, doi: 10.1038/nature11711.
- T. Francesca et al., “Exploring the Diversity of the Bifidobacterial Population in the Human Intestinal Tract,” Appl. Environ. Microbiol., vol. 75, no. 6, pp. 1534–1545, Mar. 2009, doi: 10.1128/AEM.02216-08.
- M. X. Maldonado-Gómez et al., “Stable Engraftment of Bifidobacterium longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome,” Cell Host Microbe, vol. 20, no. 4, pp. 515–526, Oct. 2016, doi: 10.1016/j.chom.2016.09.001.
- S. Fujiwara, Y. Seto, A. Kimura, and H. Hashiba, “Intestinal transit of an orally administered streptomycin–rifampicin‐resistant variant of Bifidobacterium longum SBT2928: its long‐term survival and effect on the intestinal microflora and metabolism,” J. Appl. Microbiol., vol. 90, no. 1, pp. 43–52, Jan. 2001, doi: 10.1046/j.1365-2672.2001.01205.x.
- R. Díaz, A. Torres-Miranda, G. Orellana, and D. Garrido, “Comparative Genomic Analysis of Novel Bifidobacterium longum subsp. longum Strains Reveals Functional Divergence in the Human Gut Microbiota,” Microorganisms, vol. 9, no. 9. 2021, doi: 10.3390/microorganisms9091906.
- F. Gwen, V. Angeliki, V. Kristof, and V. L. De, “Cross-Feeding between Bifidobacterium longum BB536 and Acetate-Converting, Butyrate-Producing Colon Bacteria during Growth on Oligofructose,” Appl. Environ. Microbiol., vol. 72, no. 12, pp. 7835–7841, Dec. 2006, doi: 10.1128/AEM.01296-06.
- H. Sugahara et al., “Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community,” Sci. Rep., vol. 5, no. 1, p. 13548, 2015, doi: 10.1038/srep13548.
- J. Kondo et al., “Modulatory effects of Bifidobacterium longum BB536 on defecation in elderly patients receiving enteral feeding,” World J. Gastroenterol., vol. 19, no. 14, pp. 2162–2170, 2013. https://doi.org/10.3748/wjg.v19.i14.2162.
- K. Orrhage, S. Sjöstedt, and C. E. Nord, “Effect of supplements with lactic acid bacteria and oligofructose on the intestinal microflora during administration of cefpodoxime proxetil,” J. Antimicrob. Chemother., vol. 46, no. 4, pp. 603–612, Oct. 2000, doi: 10.1093/jac/46.4.603.
- T. Odamaki, K. Kato, H. Sugahara, J. Z. Xiao, F. Abe, and Y. Benno, “Effect of probiotic yoghurt on animal-based diet-induced change in gut microbiota: an open, randomised, parallel-group study,” Benef. Microbes, vol. 7, no. 4, pp. 473–484, 2016, doi: https://doi.org/10.3920/BM2015.0173.
- H. Tamaki et al., “Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial,” Dig. Endosc., vol. 28, no. 1, pp. 67–74, Jan. 2016, doi: https://doi.org/10.1111/den.12553.
- Y. Takeda et al., “Upregulation of T-bet and tight junction molecules by Bifidobactrium longum improves colonic inflammation of ulcerative colitis,” Inflamm. Bowel Dis., vol. 15, no. 11, pp. 1617–1618, Nov. 2009, doi: 10.1002/ibd.20861.
- T. Arai, S. Mishima, S. Ohta, T. Yukioka, and T. Matsumoto, “Bifidobacterium longum BB536 and Changes in Septicemia Markers Associated with Antibiotic Use in Critically Ill Patients,” Analg. Resusc. Curr. Res., vol. 7, no. 2, 2018, doi: doi:10.4172/2324-903X.1000161.
- T. Odamaki et al., “Fluctuation of Fecal Microbiota in Individuals With Japanese Cedar Pollinosis During the Pollen Season and Influence of Probiotic Intake,” J Investig Allergol Clin Immunol, vol. 17, no. 2, pp. 92–100, 2007.
- N. Takahashi et al., “An immunostimulatory DNA sequence from a probiotic strain of Bifidobacterium longum inhibits IgE production in vitro,” FEMS Immunol. Med. Microbiol., vol. 46, no. 3, pp. 461–469, Apr. 2006, doi: 10.1111/j.1574-695X.2006.00064.x.
- T. Sakurai, T. Odamaki, and J. Xiao, “Production of Indole-3-Lactic Acid by Bifidobacterium Strains Isolated fromHuman Infants,” Microorganisms, vol. 7, no. 9. 2019, doi: 10.3390/microorganisms7090340.
- M. Xiao et al., “Cross-feeding of bifidobacteria promotes intestinal homeostasis: a lifelong perspective on the host health,” npj Biofilms Microbiomes, vol. 10, no. 1, p. 47, 2024, doi: 10.1038/s41522-024-00524-6.
- L. Oña, S. Giri, N. Avermann, M. Kreienbaum, K. M. Thormann, and C. Kost, “Obligate cross-feeding expands the metabolic niche of bacteria,” Nat. Ecol. Evol., vol. 5, no. 9, pp. 1224–1232, 2021, doi: 10.1038/s41559-021-01505-0.
- F. Turroni, C. Milani, S. Duranti, J. Mahony, D. van Sinderen, and M. Ventura, “Glycan Utilization and Cross-Feeding Activities by Bifidobacteria,” Trends Microbiol., vol. 26, no. 4, pp. 339–350, Apr. 2018, doi: 10.1016/j.tim.2017.10.001.
- S. Arboleya, C. Watkins, C. Stanton, and R. P. Ross, “Gut Bifidobacteria Populations in Human Health and Aging,” Front. Microbiol., vol. 7, 2016. https://doi.org/10.3389/fmicb.2016.01204.
- T. S. Ghosh, F. Shanahan, and P. W. O’Toole, “The gut microbiome as a modulator of healthy ageing,” Nat. Rev. Gastroenterol. Hepatol., vol. 19, no. 9, pp. 565–584, 2022, doi: 10.1038/s41575-022-00605-x.
- T. Odamaki et al., “Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study,” BMC Microbiol., vol. 16, no. 1, p. 90, 2016, doi: 10.1186/s12866-016-0708-5.
Next in Your Gut Health Journey
Explore further the fascinating world of gut health. Because better health is built one insight at a time.
Where Your Gut Meets Science
€ 269,85 Original price was: € 269,85.€ 214,95Current price is: € 214,95. incl. VAT
Consistency meets radiance. A 3-month supply of Skinbiotica COLLAGEN+—science-backed skin nutrition to support skin collagen, elasticity, and glow from within.
€ 209,90 Original price was: € 209,90.€ 179,95Current price is: € 179,95. incl. VAT
Complete beauty from within—Gutbiotica PRIME provides comprehensive microbiome support while Skinbiotica COLLAGEN+ promotes radiant and elastic skin.
€ 164,90 Original price was: € 164,90.€ 139,95Current price is: € 139,95. incl. VAT
A smart pairing for gut microbiome and weight support with Gutbiotica CORE and Fibersentials SHAPE to maintain a happy gut and support satiety naturally.







